Bioabsorbable Medical Device Design
4D’s bioabsorbable medical device designs use 4Degra© polymeric biomaterials as the basis for a new generation of implantable medical devices.

A New Approach to Bioabsorbable Medical Device Design
4D’s biomaterial science expertise and device design capabilities are powering strategic collaborations with medical device industry partners across three broad categories of bioabsorbable polymer device usage.
Utilising the unique the properties of our patented 4Degra® bioabsorbable polymer, we are currently exploring new designs and manufacturing approaches for a range of medical devices.

Design Concept | Bioabsorbable Osteotomy Wedge
Introducing 4Degra® using polycarbonate-urethane chemistry
4Degra® has been designed at the molecular level with one purpose in mind – to deliver a better bioabsorbable material for implantable medical devices.
After more than 15 years’ rigorous academic research and development, 4Degra® resins, formulated with 4D’s patented polycarbonate-urethane chemistry, is capable of creating devices that meet a wide range of medical applications.
4Degra® unlocks the potential in medical device design and manufacture
4Degra® bioabsorbable polymer is suited to a wide range of implantable medical device applications. It is a photocurable resin that can be processed into the finished device form by 3D printing, casting or conversion into thin films, meshes or microspheres.
Softer grades of 4Degra® exhibit excellent shape memory response, making them ideally suited for the manufacture of minimally invasive surgical devices such as meshes, stents, films and soft tissue scaffolds. Harder formulations are suited to more mechanically demanding uses that include trauma fixation plates, pins, screws and load-bearing bone regeneration scaffolds.
4Degra® also has potential as a drug delivery device for a range of active pharmaceutical ingredients.
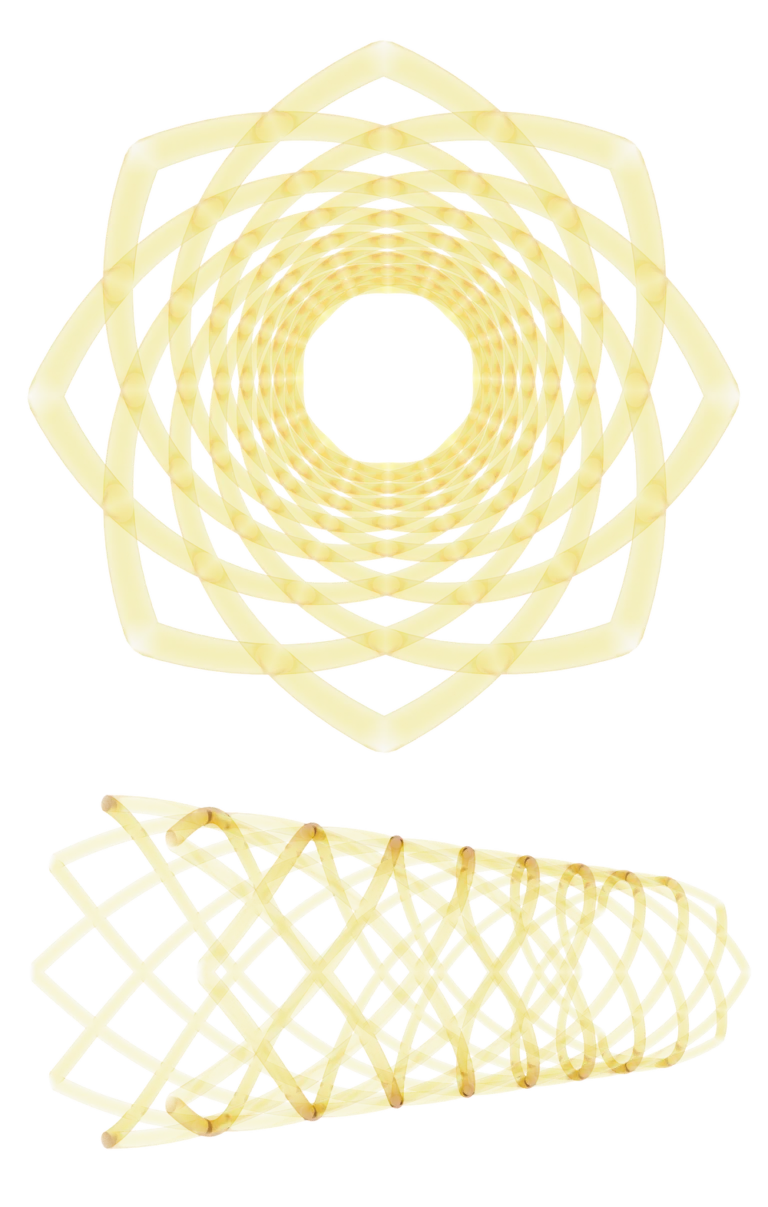
Design Concept | Bioabsorbable Stent

Musculoskeletal
Soft Tissue
Drug Delivery
In close consultation with a leading international regulatory consultancy, we are currently taking our first product, a bioabsorbable interference screw for musculoskeletal soft tissue fixation, through the U.S. FDA 510(k) regulatory pathway.
This application will facilitate a formal regulatory review of key information about the safety, biocompatibility and manufacturability of an implantable device composed of 4Degra®, creating a reference for future device regulatory applications.
This lead product is anticipated to receive FDA 510(k) clearance in early 2026 and will provide a technological, regulatory and commercial bridgehead into the musculoskeletal market.

Development Product | Bioabsorbable Interference Screw
REGULATORY DISCLAIMER
The medical devices and product concepts featured on this website and in other corporate communication materials are for informational purposes only and are not currently cleared, approved, or authorized for marketing or sale in any geography or jurisdiction. Any references to potential applications or benefits are based on ongoing research and development efforts. Regulatory review and clearance or approval are required before these products can be made available for clinical use. Nothing on this site should be construed as a guarantee of future availability or regulatory clearance or approval.
Future product pipeline
In collaboration with our industry partners, 4D is exploring medical device applications for novel surgical solutions to challenging problems in high-value, high-growth segments across musculoskeletal, soft tissue and drug delivery categories.
Strategic partnerships
Alongside internal product development efforts, 4D is engaging with proven sales channel partners to explore synergistic opportunities to launch device innovations into key market segments and geographies.
If you would like to discuss future product innovations or explore new strategic partnerships, please do get in touch.
